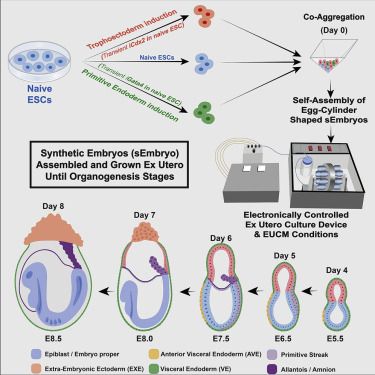
Post-gastrulation synthetic embryos generated ex utero from mouse naive ESCs
Highlights
- •Advanced synthetic embryos (sEmbryos) self-assembled from ESCs in an ex utero setup
- •Naive ESCs give rise to all embryonic and extraembryonic compartments in sEmbryos
- •Post-gastrulation stem cell derived sEmbryos develop organ-specific progenitors
- •Extraembryonic compartments adequately develop in post-gastrulation whole sEmbryos
Summary
Graphical abstract
Keywords
Introduction
Results
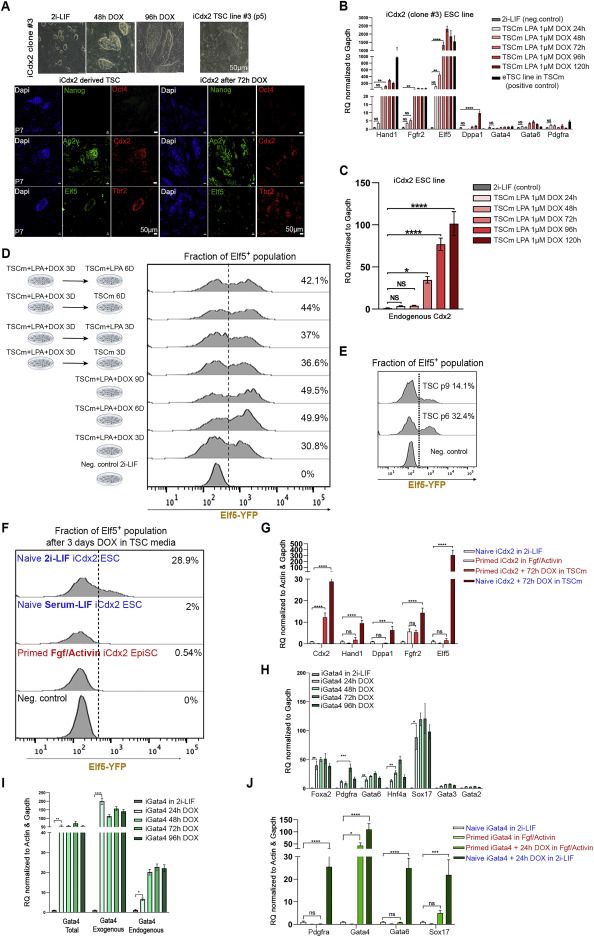
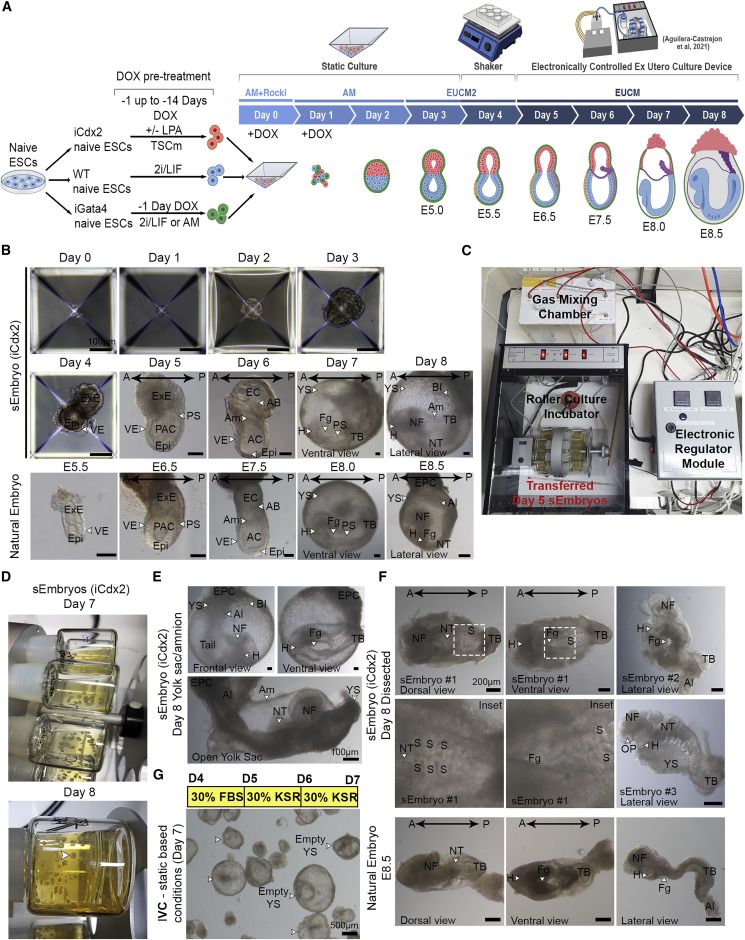
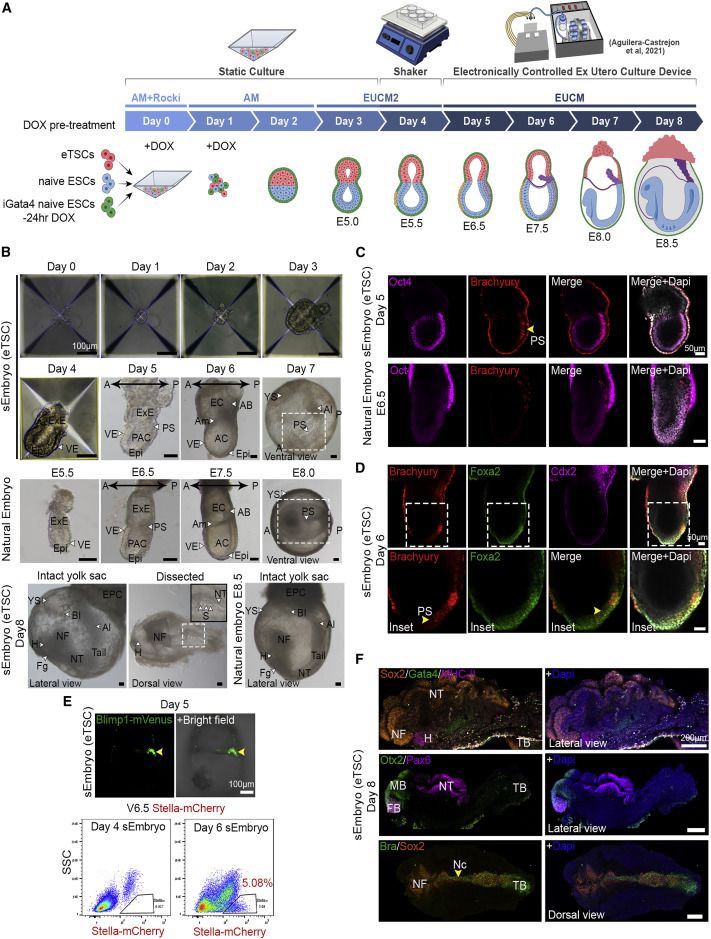
Egg-cylinder-shaped sEmbryos generated solely from naive ESCs
sEmbryos self-assembled from naive ESCs develop ex utero up to early organogenesis
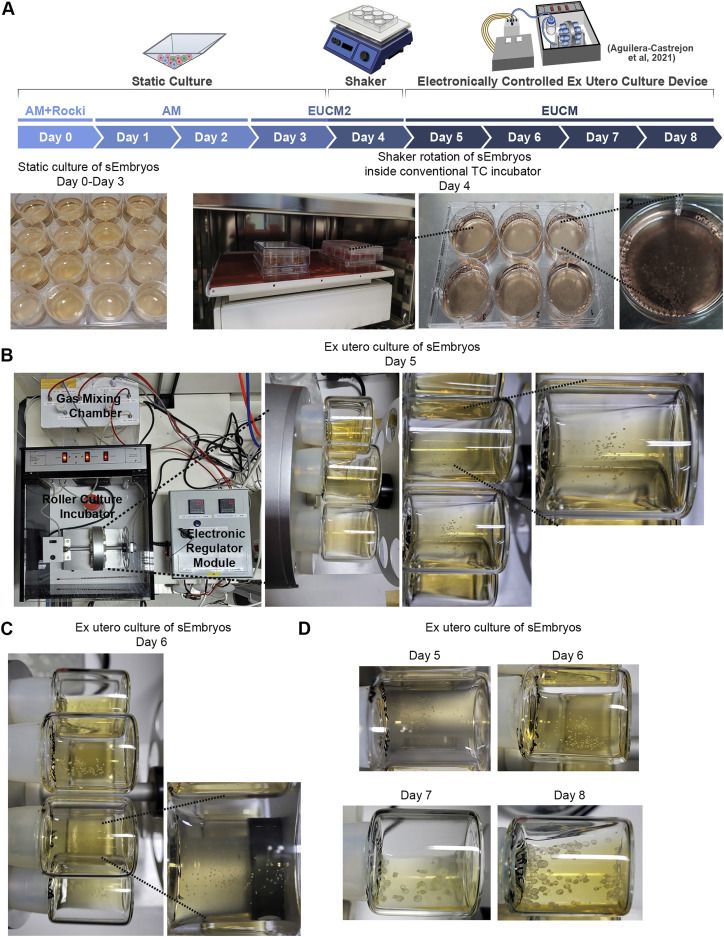
share ');background-repeat:no-repeat;background-size:contain;width:100%;height:100%;background-position:50% 50%;"> Video S1. Advanced mouse synthetic embryos grown in electronically controlled ex utero platform and conditions, related to Figure 2Electronically regulated roller culture incubator with customized gas concentration and pressure regulation module was utilized for growing mouse synthetic embryos as shown. Rotating glass bottles showing advanced mouse synthetic embryos (iCdx2 sEmbryos) at day 8 of ex utero culture protocol for synthetic embryos (as shown in Figure 2D).
share ');background-repeat:no-repeat;background-size:contain;width:100%;height:100%;background-position:50% 50%;"> Video S2. E8.5 equiv post-gastrulation synthetic mouse embryos, related to Figure 2Part 1- Representative example of an undissected Day 8 synthetic embryo (iCdx2) within its extraembryonic compartments. Day 8 synthetic embryo (iCdx2) is showing beating heart, neural folds, allantois, vitelline circulation and ectoplacental cone. Part 2- Representative examples of dissected day 8 synthetic embryos after removal of extraembryonic tissues. Dissected Day 8 synthetic embryos are showing a beating heart, the neural tube and neural folds. Part 3 - Representative example of an undissected Day 8 synthetic embryo (eTSC) highlighting blood islands in yolk sac and the ectoplacental cone. Day 8 synthetic embryo (eTSC) is showing vitelline circulation, blood islets formation in yolk sac, a beating heart and an ectoplacental cone.
share ');background-repeat:no-repeat;background-size:contain;width:100%;height:100%;background-position:50% 50%;"> Video S3. Mouse sEmbryos generated solely from naive ESCs ex utero, related to Figure 2Representative video captions showing different steps in generating sEmbryos until day 5 of the protocol (before the sEmbryos are transferred to the electronically controlled ex utero roller culture platform).
share ');background-repeat:no-repeat;background-size:contain;width:100%;height:100%;background-position:50% 50%;"> Video S4. Setting up electronically controlled ex utero roller culture platform to grow post-gastrulation sEmbryos, related to Figure 2Representative video captions showing different steps in setting up the electronically controlled ex utero roller culture platform in preparation for transferring naive ESC derived day 5 sEmbryos.
share ');background-repeat:no-repeat;background-size:contain;width:100%;height:100%;background-position:50% 50%;"> Video S5. Handling of sEmbryos grown in electronically controlled ex utero roller culture platform, related to Figure 2Representative video captions showing different steps in transferring and handling sEmbryos grown in electronically controlled ex utero roller culture platform and EUCM conditions from day 5–8.
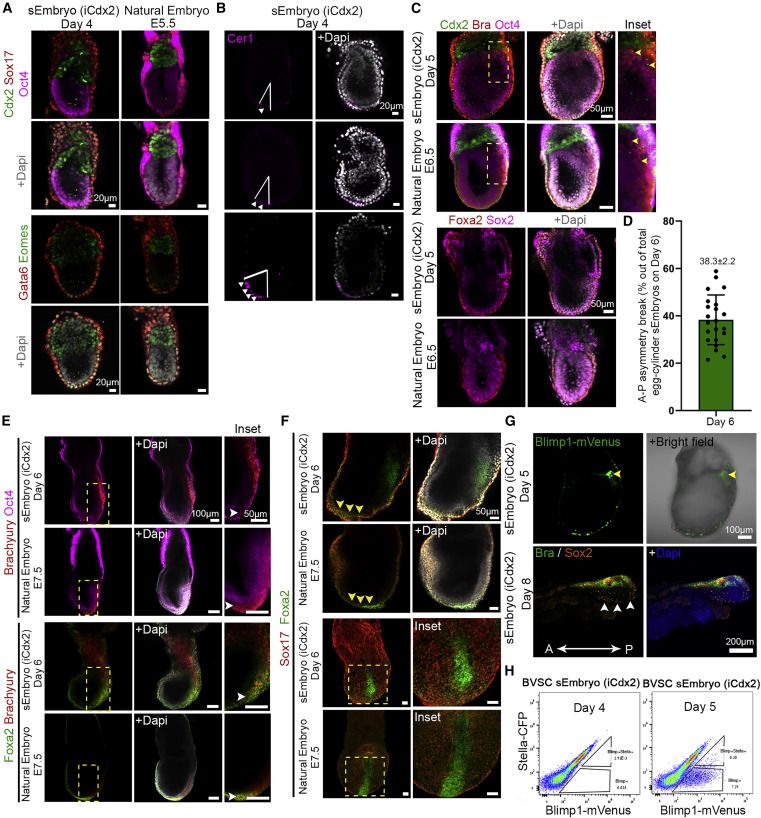
sEmbryos recapitulate morphological changes occurring during natural embryo development
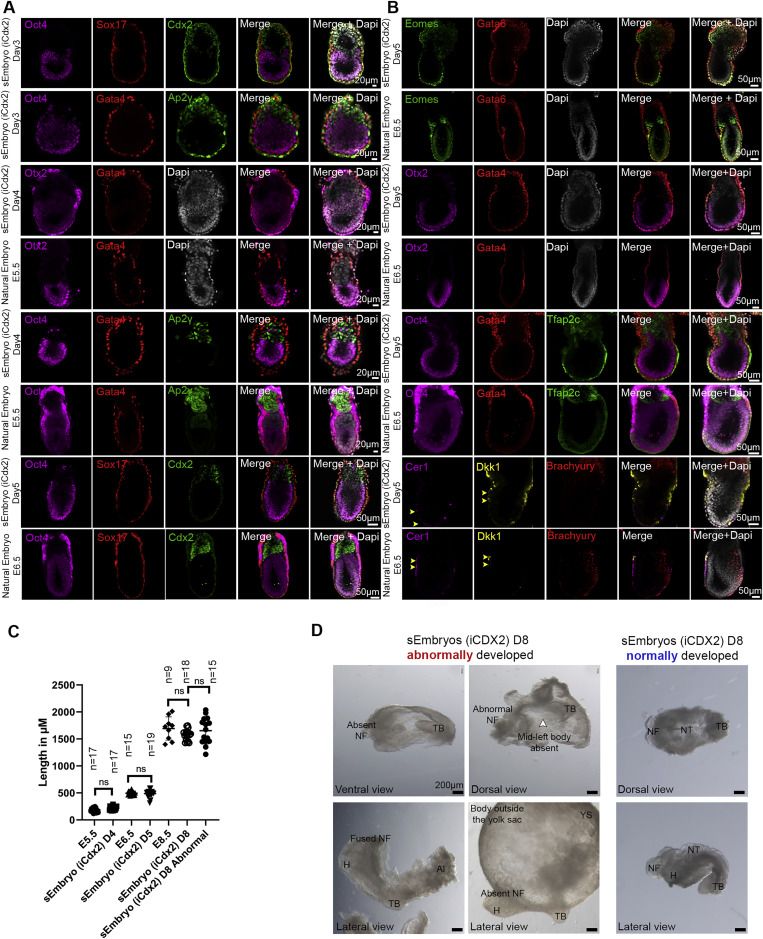
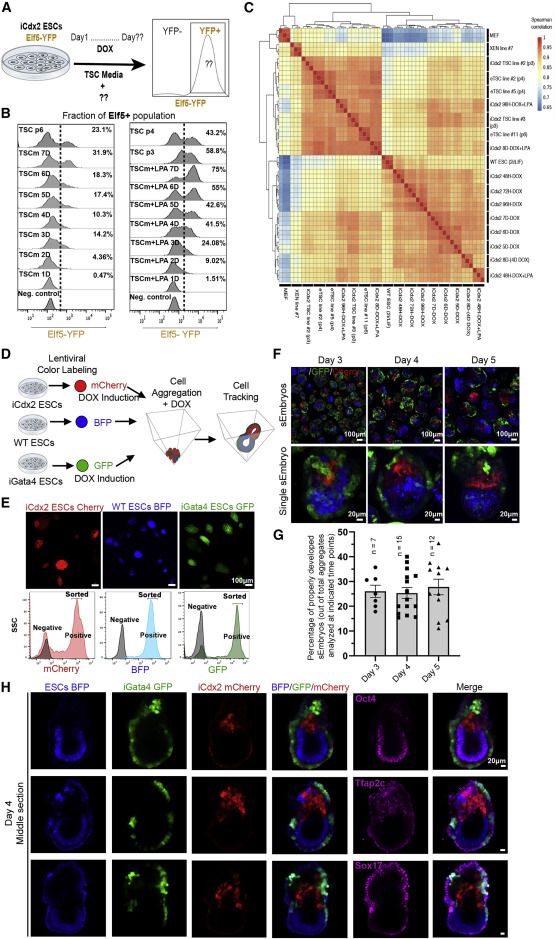
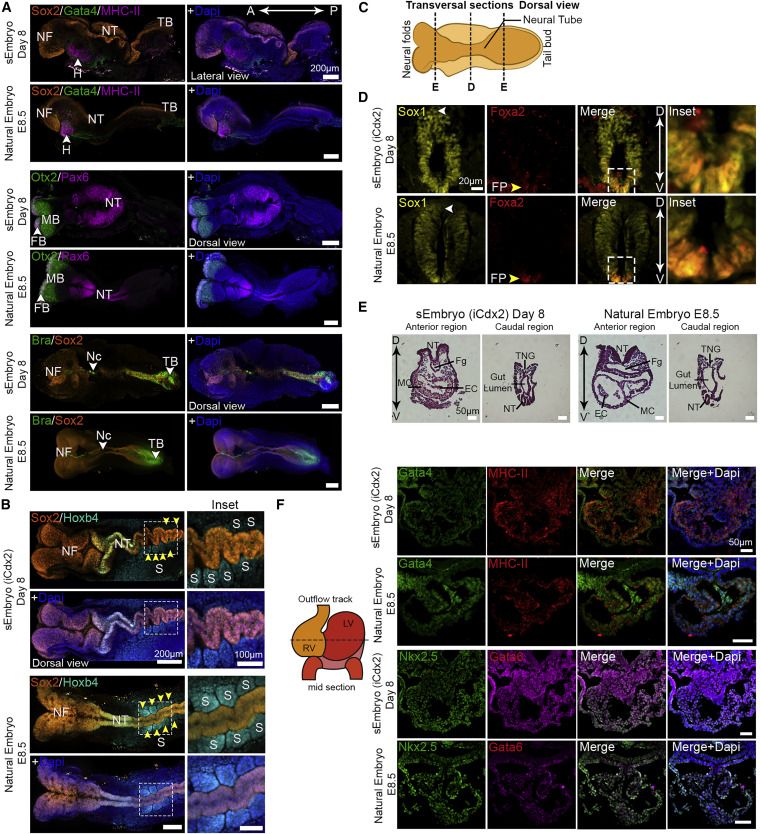
Adequate spatiotemporal expression of lineage markers in advanced sEmbryos
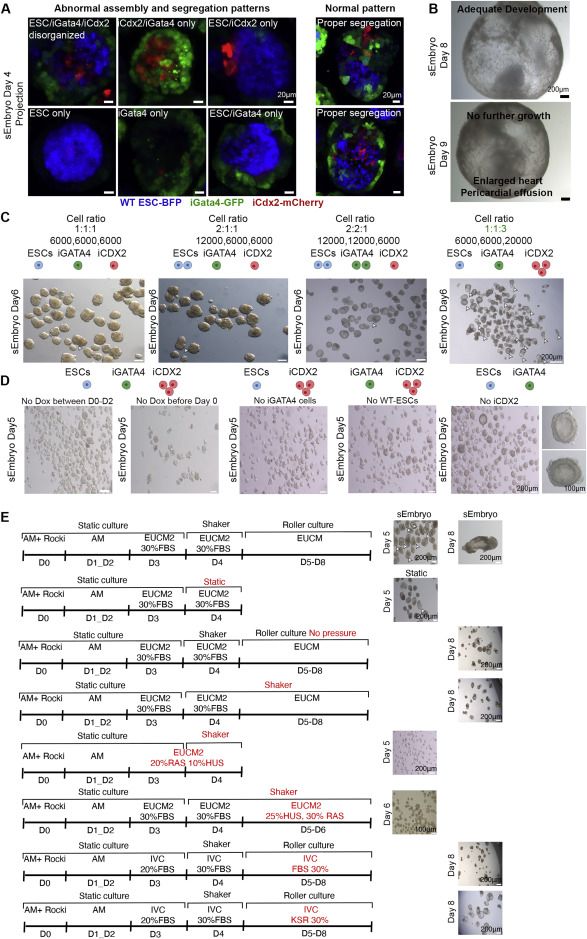
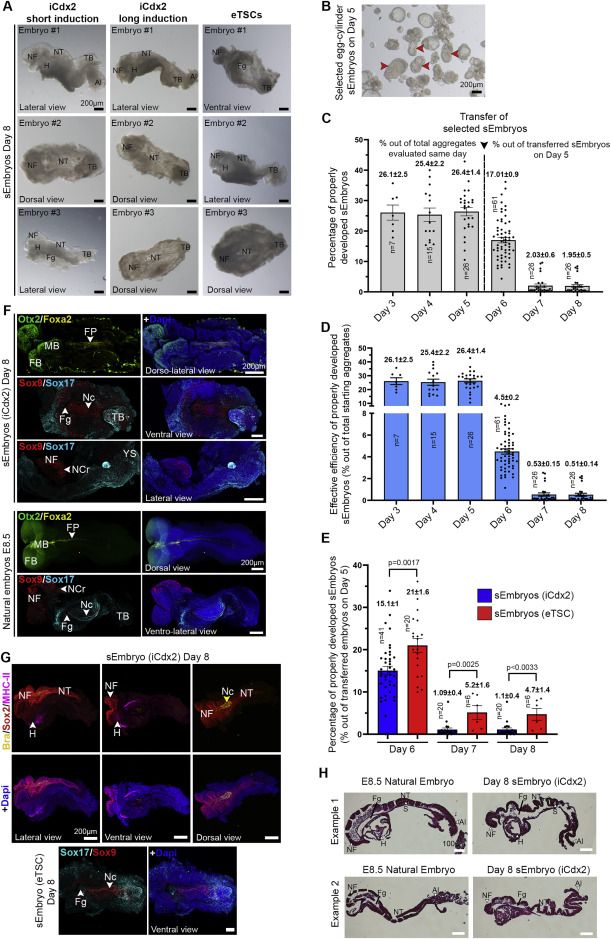
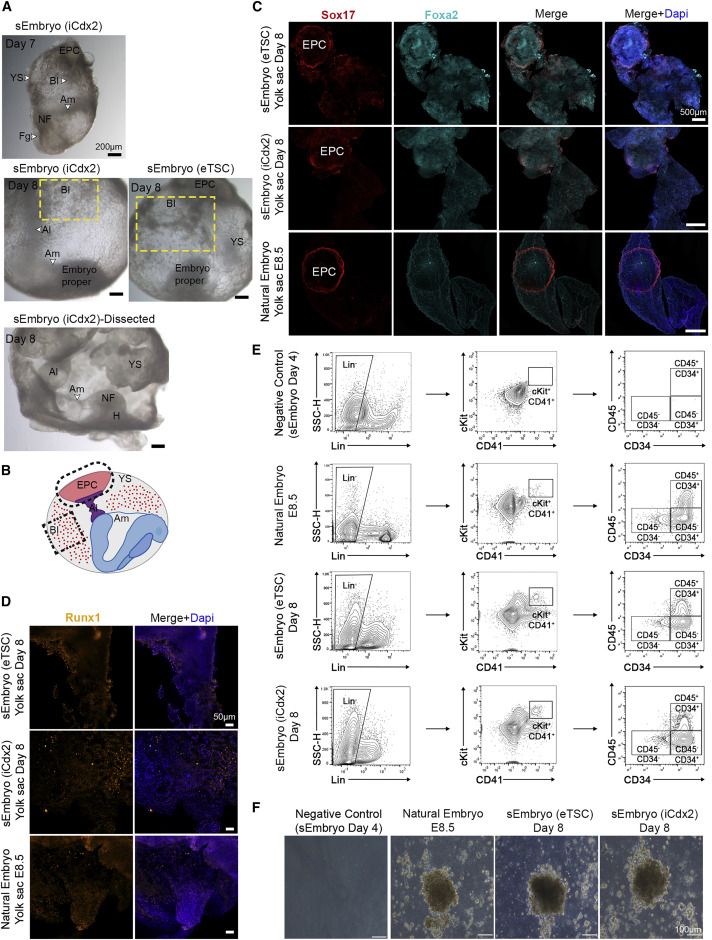
Embryo-derived TSC lines can propel self-organization in advanced sEmbryos
Development of extraembryonic compartments in post-gastrulation sEmbryos
scRNA-seq analysis validates mouse sEmbryo complexity
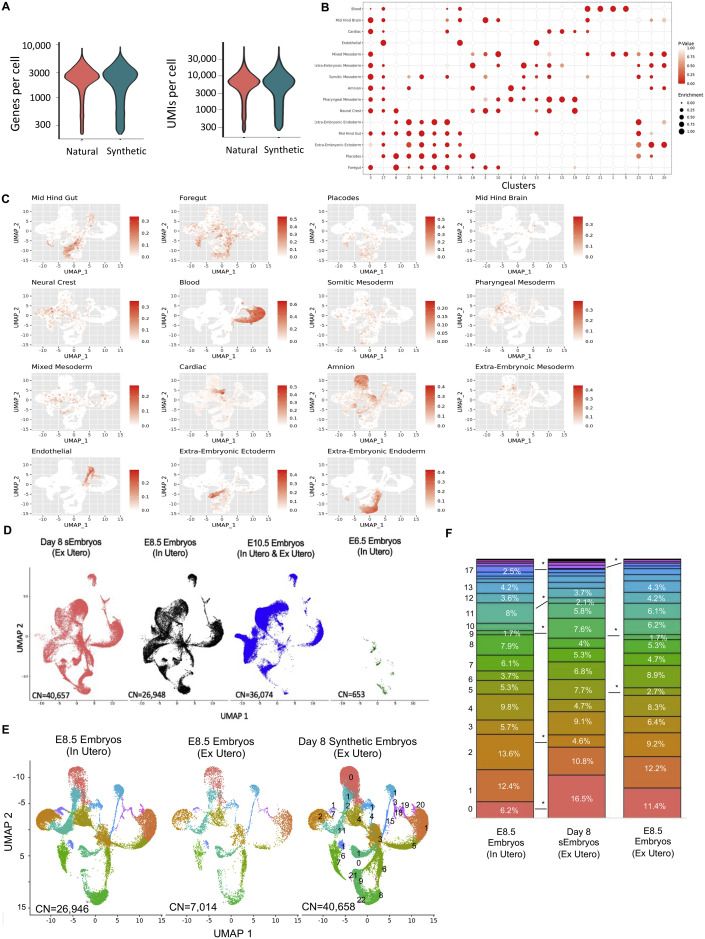
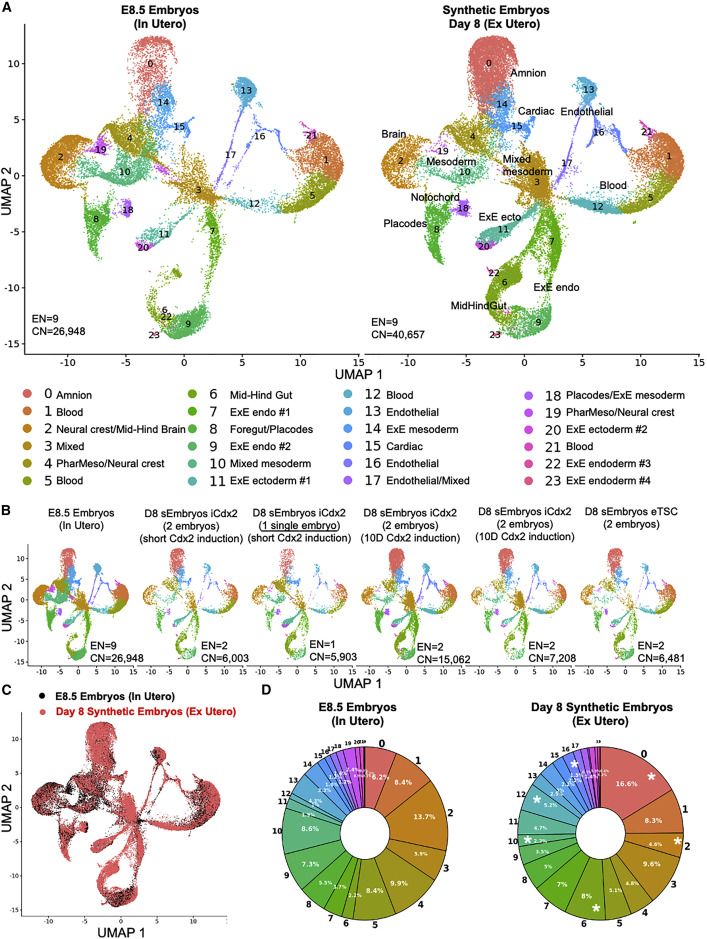
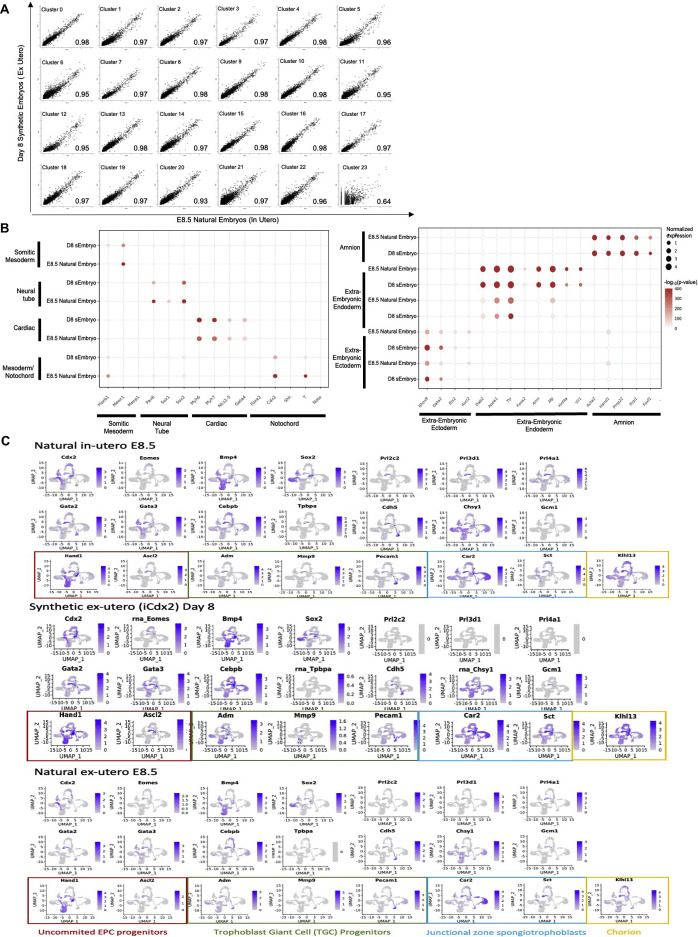
Discussion
Limitations of the study
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit anti-Gata4 (1:200) | Abcam | Cat# Ab84593; RRID: AB_10670538 |
| Rabbit anti-Foxa2 (1:100) | Abcam | Cat# Ab40874; RRID: AB_732411 |
| Goat anti-Sox2 (1:200) | R&D | Cat# AF2018; RRID: AB_355110 |
| Mouse Anti-Myosin Heavy Chain 2 (1:100) | R&D | Cat# MAB4470; Clone# MF-20; RRID: AB_1293549 |
| Goat anti-Sox17 (1:100) | R&D | Cat# AF1924; RRID: AB_355060 |
| Rabbit anti-Brachyury (1:100) | Cell Signaling | Cat# 81694; RRID: AB_2799983 |
| Rabbit anti-Cdx2 (1:100) | Abcam | Cat# Ab76541; RRID: AB_1523334 |
| Mouse anti-Oct3/4 (1:100) | Santa Cruz | Cat# Sc-5279; RRID: AB_628051 |
| Goat anti-Otx2 (1:100) | R&D | Cat# AF1979; RRID: AB_2157172 |
| Goat anti-Tfap2c/Ap2γ (1:100) | R&D | Cat# AF5059; RRID: AB_2255891 |
| Goat anti-Gata6 (1:100) | R&D | Cat# AF1700; RRID: AB_2108901 |
| Rabbit anti-Eomes/Tbr2 (1:50) | Abcam | Cat# Ab23345; RRID: AB_778267 |
| Rat anti-Cerberus 1 (1:100) | R&D | Cat# MAB1986; RRID: AB_2275974 |
| Rabbit anti-Sox9 (1:100) | Millipore | Cat# Ab5535; RRID: AB_2239761 |
| Mouse anti-Gata3 (1:100) | Invitrogen | Cat# MA1-028; RRID: AB_2536713 |
| Rabbit anti-Nanog (1:100) | Bethyl | Cat# AF1997; RRID: AB_355097 |
| Goat anti-Elf5 (1:100) | Santa Cruz | Cat# Sc-9645; RRID: AB_640106 |
| Goat anti-Dkk1 (1:100) | R&D | Cat# AF1096; RRID: AB_354597 |
| Goat anti-Brachyury (1:100) | R&D | Cat# AF2085; RRID: AB_2200235 |
| Rabbit anti-Nkx2.5 (1:100) | Abcam | Cat# Ab97355; RRID: AB_10680260 |
| Rabbit anti-Hoxb4 (1:100) | Abcam | Cat#: ab133521; Clone: ERP1917 |
| Rabbit anti-Pax6 (1:100) | Biolegend | Cat#: 901301; RRID: AB_2565003 |
| Rabbit anti-Runx1 (1:100) | Abcam | Cat#: ab240639; Clone: ERP23044-100 |
| Rat anti-cKit (1:50) | Biolegend | Cat# 105802; RRID: AB_313211 |
| Rat anti-CD45 (1:100) | Biolegend | Cat# 103102; RRID: AB_312967 |
| Rat anti-CD41 (1:50) | Biolegend | Cat# 133906; RRID: AB_2129745 |
| Rat anti-CD34 (1:50) | eBioscience | Cat# 14-0341-82; RRID: AB_467210 |
| Streptavidin-PE-Cy7 | Biolegend | Cat# 405206; |
| Mouse Lineage cell detection cocktail-biotin (1:20) | Miltenyi | Cat#130-092-613; RRID: AB_1103214 |
| Alexa Fluor 488-conjugated AffiniPure Donkey anti-rabbit IgG (H + L) | Jackson | Cat# 711-545-152; RRID: AB_2313584 |
| Alexa Fluor 488-conjugated AffiniPure Donkey anti-Goat IgG (H + L) | Jackson | Cat# 705-545-003; RRID: AB_2340428 |
| Rhodamine-Red-X-conjugated AffiniPure Donkey anti-Rabbit IgG (H + L) | Jackson | Cat# 711-295-152; RRID: AB_2340613 |
| Rhodamine-Red-X-conjugated AffiniPure Donkey anti-Gaot IgG (H + L) | Jackson | Cat# 705-295-003; RRID: AB_2340422 |
| Rhodamine-Red-X-conjugated AffiniPure Donkey anti-Mouse IgG (H + L) | Jackson | Cat# 715-295-150; RRID: AB_2340831 |
| Alexa Fluor 647-conjugated AffiniPure Donkey anti-rabbit IgG (H + L) | Jackson | Cat# 711-605-152; RRID: AB_2492288 |
| Alexa Fluor 647-conjugated AffiniPure Donkey anti-Goat IgG (H + L) | Jackson | Cat# 705-605-003; RRID: AB_2340436 |
| Alexa Fluor 647-conjugated AffiniPure Donkey anti-Mouse IgG (H + L) | Jackson | Cat# 715-605-150; RRID: AB_2340862 |
| Alexa Fluor 647-conjugated AffiniPure Donkey anti-Rat IgG (H + L) | Jackson | Cat# 712-545-153; RRID: AB_2340684 |
| Chemicals, peptides, and recombinant proteins | ||
| FGF4 | Peprotech | 100–31 |
| Heparin | Sigma | H3149 |
| 0.25% Trypsin-EDTA | Biological industries-Sartorius | 03-050-1B |
| 0.05% trypsin-EDTA | Biological industries-Sartorius | 03-053-1B |
| DMEM Medium | GIBCO | 41965 |
| FBS Serum (for growing MEFs and ES lines) | GIBCO | 10270–106 |
| GlutaMAX | GIBCO | 35050061 |
| Penicillin streptomycin | Biological industries-Sartorius | 03-031-1B |
| Sodium Pyruvate | Biological industries-Sartorius | 03-042-1B |
| Non-essential amino acids | Biological industries-Sartorius | 01-340-1B |
| β-Mercaptoethanol | Thermo | 31350010 |
| Recombinant Human LIF | This study | N/A |
| Neurobasal | Thermo | 21103049 |
| DMEM-F12 with HEPES Medium | Sigma | D6421 |
| N2 | Invitrogen | 17502048 |
| B27 | Invitrogen | 17504044 |
| CHIR99021 | Axon Medchem | 1386 |
| PD0325901 | Axon Medchem | 1408 |
| RPMI1640 | GIBCO | 21875 |
| FBS (for sEmbryo aggregation stages) | Sigma | F7524 |
| Doxycycline | Sigma | D9891 |
| ROCKi Y27632 | Axon Medchem | 1683 |
| LPA (Oleoyl-L-α-lysophosphatidic acid) | Sigma | L7260 |
| Advanced DMEM/F12 | GIBCO | 21331–020 |
| D(+)-Glucose Monohydrate | J.T. Baker | 0113 |
| T3 (3,3′,5-Triiodo-L-thyronine sodium salt) | Sigma-Aldrich | T6397 |
| HEPES | GIBCO | 15630056 |
| CMRL | GIBCO | 11530037 |
| ITS-X | Thermo Fisher Scientific | 51500–056 |
| B-estradiol | Sigma | E8875 |
| Progesterone | Sigma | P0130 |
| N-acetyl-L-cysteine | Sigma | A7250 |
| DMEM W/O phenol red W/O L-glutamine Medium | GIBCO | 11880 |
| Rat Serum | ENVIGO Bioproducts | B-4520 |
| MethoCult | Stem Cell Technologies | SF M3436 |
| Normal Donkey Serum | Jackson Laborratories | 017-000-121 |
| Critical commercial assays | ||
| Syber Green PCR master Mix | Thermo Fisher | 4385614 |
| JetPEI Transfection Reagent | Polyplus Inc. | 101-10N |
| Deposited data | ||
| scRNA-seq data E8.5 natural ex Utero | GSE149372 | |
| scRNA-seq data | This study | GSE208681 |
| Bulk MARS RNA- seq data | This study | GSE208681 |
| Additional supplementary technical data and results | This study | Mendeley Data: https://doi.org/10.17632/6nhpgnxf3y.1 |
| Experimental models: Cell lines | ||
| Mouse: BVSC ESC (Blimp1-mVenus-Stella-CFP ESCs) | N/A | |
| Mouse: V6.5 WT ESCs | N/A | |
| Mouse: KH2 WT ESCs | N/A | |
| Mouse: ICR1 ESCs | This study | N/A |
| Mouse: BDF2 TSCs (clone #2 and #5) | This study | N/A |
| Mouse: ICR XEN (clone #7) | This study | N/A |
| Mouse: KH2-Cdx2 ESCs (clone #3) (iCdx2) | This study | N/A |
| Mouse: KH2-Cdx2-Elf5-EYFP (clone #6) | This study | N/A |
| Mouse: KH2-Gata4 ESCs (clone #7) (iGata4) | This study | N/A |
| Human: HEK293T | ATCC | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: BDF1 | ENVIGO | N/A |
| Mouse: 129sJae | ENVIGO | N/A |
| Mouse: ICR | ENVIGO | N/A |
| Oligonucleotides | ||
| Data S2 | This study | N/A |
| Recombinant DNA | ||
| TetO-Cdx2 flip-in construct | This study | N/A |
| TetO-Gata4 flip-in construct | This study | N/A |
| Elf5-EYFP targeting vector | Addgene 128833 | |
| pRSV-Rev | Kind gift from Gustavo Mostolavsky lab (Boston University School of Medicine, USA) | N/A |
| pMDLg/pRRE | Kind gift from Gustavo Mostolavsky lab (Boston University School of Medicine, USA) | N/A |
| pMD2.G | Kind gift from Gustavo Mostolavsky lab (Boston University School of Medicine, USA) | N/A |
| pHAGE zsGreen | Kind gift from Gustavo Mostolavsky lab (Boston University School of Medicine, USA) | N/A |
| pHAGE tdTomato | Kind gift from Gustavo Mostolavsky lab (Boston University School of Medicine, USA) | N/A |
| pHAGE TagBFP | Kind gift from Gustavo Mostolavsky lab (Boston University School of Medicine, USA) | N/A |
| Software and algorithms | ||
| FlowJo | FlowJo, LLC | https://www.flowjo.com/ |
| ZEN software | Zeiss | https://www.zeiss.com/microscopy/int/products/microscope-software/zen-lite.html |
| Prism8 | GraphPad Inc. | https://www.graphpad.com/scientific-software/prism/ |
| ImageJ | NIH, USA | https://imagej.nih.gov/ij/ |
| CellSens Entry | Olympus | https://www.olympus-lifescience.com/en/software/cellsens/ |
| 10X Genomics CellRanger 7.0 | 10X Genomics Inc. | https://support.10xgenomics.com/single-cell-gene-expression/software/downloads/latest |
| Seurat R package v3.2.2 | Satija Lab, USA | https://satijalab.org/seurat/ |
| AUCell R package v1.8 | Bioconductor project, USA | https://bioconductor.org/packages/release/bioc/html/AUCell.html |
| UTAP | Bioinformatics unit, Weizmann Institute of Science, Israel | https://utap.readthedocs.io/en/latest/ |
| DESeq2.0 | Bioconductor project, USA | https://bioconductor.org/packages/release/bioc/html/DESeq2.html |
| IGV | Broad Institute, USA | https://software.broadinstitute.org/software/igv/ |
| Other | ||
| PVDF filter 0.22 μm | Millipore | SLGV033RS |
| 35mm glass bottom dishes | In Vitro Scientific | D35201.5N |
| 0.22 μM filter | Nalgene | 565–0020 |
| AggreWell 24-well plate 400 | STEMCELL Technologies | 34415 |
| AggreWell 24-well plate 800 | STEMCELL Technologies | 34815 |
| Anti-adherent rinsing solution | STEMCELL Technologies | 07010 |
| 6-well cell suspension non adherent culture plate | Greiner Bio | 657185 |
| LSM 700 inverted confocal microscope | Zeiss | N/A |
| Shaker (placed inside tissue culture incubator) | Thermo Scientific | 88881102 and 88881123 |
| Electronic controller unit and gas mixing box for roller culture system | Hanna lab model 1 and 1.2 (assembled by Arad Technologies Ltd) | |
| Roller Culture Incubator | Cullum Starr Ltd. | BTC Precision Incubator |
Resource availability
Lead contact
Materials availability
Experimental model and subject details
Animals
Stem cell lines
Method details
Naive ESC and other stem cell line in vitro culture conditions
Generation of iGata4 and iCdx2 ESCs clones
Generation of Elf5-EYFP reporter iCdx2 ESCs
Generation of fluorescent labeled ESCs
Human umbilical cord serum (HUS) and human adult serum (HAS)
Electronically controlled ex utero roller culture platform
Naive ESC-derived synthetic embryo ex utero culture
Whole-mount immunostaining of sEmbryos
Immunohistochemistry and histological analysis
Confocal microscopy
Morphological evaluation of mouse early development and efficiency calculations
Assessment of sEmbryo length
Yolk sac erythroid progenitor staining
Erythroid colony forming assay
RNA extraction & RT-PCR analysis
Bulk RNA-seq (Bulk MARS-seq)
Bulk RNA-seq analysis
10X single cell RNA-seq
10X single cell RNA-seq analysis
Quantification and statistical analysis
Data and code availability
- •All bulk and scRNA-seq data are deposited under GEO: GSE208681.
- •Related additional figures and data can be found on Mendeley Data (https://doi.org/10.17632/6nhpgnxf3y.1).
- •This paper does not report original code. Codes used to analyze the RNA-sequencing data are available from the authors upon request. Any additional information required to reanalyze the data reported in this work is available from the lead contact upon request.
Acknowledgments
Author contributions
Declaration of interests
Inclusion and diversity
Supplemental information
- Data S1. Supplementary Spreadsheet 1: RNA-seq sample information and selected normalized expression patterns, related to Figures 1 and 7
Sample information of X platform based single-cell RNA-seq samples. Sample information of bulk 3′UTR RNA-seq samples. Normalized expression values measured by bulk 3′UTR RNA-seq during induction of ESCs toward trophectoderm lineage and TSCs (eTSCs).
- Data S2. Supplementary Spreadsheet 2 – Sequence of PCR primers used in this study, related to STAR Methods
References
- Aguilera-Castrejon A.
- Oldak B.
- Shani T.
- Ghanem N.
- Itzkovich C.
- Slomovich S.
- Tarazi S.
- Bayerl J.
- Chugaeva V.
- Ayyash M.
- et al.
Ex utero mouse embryogenesis from pre-gastrulation to late organogenesis.Nature. 2021; 593: 119-124https://doi.org/10.1038/s41586-021-03416-3- Amadei G.
- Lau K.Y.
- de Jonghe J.
- Gantner C.W.
- Sozen B.
- Chan C.
- Zhu M.
- Kyprianou C.
- Hollfelder F.
- Zernicka-Goetz M.
Inducible stem-cell-derived embryos capture mouse morphogenetic events in vitro.Dev. Cell. 2021; 56: 366-382.e9https://doi.org/10.1016/j.devcel.2020.12.004- Anderson K.G. v
- Hamilton W.B.
- Roske F. v
- Azad A.
- Knudsen T.E.
- Canham M.
- Forrester L.M.
- Brickman J.M.
Insulin fine-tunes self-renewal pathways governing naive pluripotency and extra-embryonic endoderm.Nat. Cell Biol. 2017; 19: 1164-1177https://doi.org/10.1038/ncb3617- Bayerl J.
- Ayyash M.
- Shani T.
- Manor Y.S.
- Gafni O.
- Massarwa R.
- Kalma Y.
- Aguilera-Castrejon A.
- Zerbib M.
- Amir H.
- et al.
Principles of signaling pathway modulation for enhancing human naive pluripotency induction.Cell Stem Cell. 2021; 28: 1549-1565.e12https://doi.org/10.1016/j.stem.2021.04.001- Beccari L.
- Moris N.
- Girgin M.
- Turner D.A.
- Baillie-Johnson P.
- Cossy A.-C.
- Lutolf M.P.
- Duboule D.
- Arias A.M.
Multi-axial self-organization properties of mouse embryonic stem cells into gastruloids.Nature. 2018; 562: 272-276https://doi.org/10.1038/s41586-018-0578-0- Bedzhov I.
- Zernicka-Goetz M.
Self-organizing properties of mouse pluripotent cells initiate morphogenesis upon implantation.Cell. 2014; 156: 1032-1044https://doi.org/10.1016/j.cell.2014.01.023- Behringer R.
- Gertsenstein M.
- Nagy K.V.
- Nagy A.
Chapter 5: Isolation, culture and manipulation of post-implantation embryos.Manipulating the Mouse Embryo. Fourth Edition. Cold Spring Harbor Laboratory Press, 2014: 152-153- Benchetrit H.
- Jaber M.
- Zayat V.
- Sebban S.
- Pushett A.
- Makedonski K.
- Zakheim Z.
- Radwan A.
- Maoz N.
- Lasry R.
- et al.
Direct induction of the three pre-implantation blastocyst cell types from fibroblasts.Cell Stem Cell. 2019; 24: 983-994.e7https://doi.org/10.1016/J.STEM.2019.03.018- Blij S.
- Parenti A.
- Tabatabai-Yazdi N.
- Ralston A.
Cdx2 efficiently induces trophoblast stem-like cells in naive, but not primed, pluripotent stem cells.Stem Cell. Dev. 2015; 24: 1352-1365https://doi.org/10.1089/scd.2014.0395- Bradley A.
- Evans M.
- Kaufman M.H.
- Robertson E.
Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines.Nature. 1984; 309: 255-256https://doi.org/10.1038/309255a0- Cambuli F.
- Murray A.
- Dean W.
- Dudzinska D.
- Krueger F.
- Andrews S.
- Senner C.E.
- Cook S.J.
- Hemberger M.
Epigenetic memory of the first cell fate decision prevents complete ES cell reprogramming into trophoblast.Nat. Commun. 2014; 5: 5538https://doi.org/10.1038/ncomms6538- Chan M.M.
- Smith Z.D.
- Grosswendt S.
- Kretzmer H.
- Norman T.M.
- Adamson B.
- Jost M.
- Quinn J.J.
- Yang D.
- Jones M.G.
- et al.
Molecular recording of mammalian embryogenesis.Nature. 2019; 570: 77-82https://doi.org/10.1038/s41586-019-1184-5- Choi J.
- Huebner A.J.
- Clement K.
- Walsh R.M.
- Savol A.
- Lin K.
- Gu H.
- di Stefano B.
- Brumbaugh J.
- Kim S.Y.
- et al.
Prolonged Mek1/2 suppression impairs the developmental potential of embryonic stem cells.Nature. 2017; 548: 219-223https://doi.org/10.1038/nature23274- De Paepe C.
- Cauffman G.
- Verloes A.
- Sterckx J.
- Devroey P.
- Tournaye H.
- Liebaers I.
- Van de Velde H.
Human trophectoderm cells are not yet committed.Human Reproduction. 2013; 28: 740-749https://doi.org/10.1093/humrep/des432- Fujikura J.
- Yamato E.
- Yonemura S.
- Hosoda K.
- Masui S.
- Nakao K.
- Miyazaki J.i.
- Niwa H.
Differentiation of embryonic stem cells is induced by GATA factors.Genes Dev. 2002; 16: 784-789https://doi.org/10.1101/gad.968802- Gafni O.
- Weinberger L.
- Mansour A.A.
- Manor Y.S.
- Chomsky E.
- Ben-Yosef D.
- Kalma Y.
- Viukov S.
- Maza I.
- Zviran A.
- et al.
Derivation of novel human ground state naive pluripotent stem cells.Nature. 2013; 504: 282-286https://doi.org/10.1038/nature12745- Gardner R.L.
- Papaioannou V.E.
- Barton S.C.
Origin of the ectoplacental cone and secondary giant cells in mouse blastocysts reconstituted from isolated trophoblast and inner cell mass.J Embryol Exp Morphol. 1973; 30: 561-572https://doi.org/10.1242/DEV.30.3.561- Hanna J.
- Markoulaki S.
- Mitalipova M.
- Cheng A.W.
- Cassady J.P.
- Staerk J.
- Carey B.W.
- Lengner C.J.
- Foreman R.
- Love J.
- et al.
Metastable pluripotent states in NOD-mouse-derived ESCs.Cell Stem Cell. 2009; 4: 513-524https://doi.org/10.1016/j.stem.2009.04.015- Harrison S.E.
- Sozen B.
- Christodoulou N.
- Kyprianou C.
- Zernicka-Goetz M.
Assembly of embryonic and extraembryonic stem cells to mimic embryogenesis in vitro.Science. 2017; 356: eaal1810https://doi.org/10.1126/science.aal1810- Hayashi K.
- Ohta H.
- Kurimoto K.
- Aramaki S.
- Saitou M.
Reconstitution of the mouse germ cell specification pathway in culture by pluripotent stem cells.Cell. 2011; 146: 519-532https://doi.org/10.1016/j.cell.2011.06.052- Hochedlinger K.
- Yamada Y.
- Beard C.
- Jaenisch R.
Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues.Cell. 2005; 121: 465-477https://doi.org/10.1016/j.cell.2005.02.018- Ibarra-Soria X.
- Jawaid W.
- Pijuan-Sala B.
- Ladopoulos V.
- Scialdone A.
- Jörg D.J.
- Tyser R.C.V.
- Calero-Nieto F.J.
- Mulas C.
- Nichols J.
- et al.
Defining murine organogenesis at single-cell resolution reveals a role for the leukotriene pathway in regulating blood progenitor formation.Nat. Cell Biol. 2018; 20: 127-134https://doi.org/10.1038/s41556-017-0013-z- Iturri L.
- Freyer L.
- Biton A.
- Dardenne P.
- Lallemand Y.
- Gomez Perdiguero E.
Megakaryocyte production is sustained by direct differentiation from erythromyeloid progenitors in the yolk sac until midgestation.Immunity. 2021; 54: 1433-1446.e5https://doi.org/10.1016/j.immuni.2021.04.026- Kagawa H.
- Javali A.
- Khoei H.H.
- Sommer T.M.
- Sestini G.
- Novatchkova M.
- Scholte Op Reimer Y.
- Castel G.
- Bruneau A.
- Maenhoudt N.
- et al.
Human blastoids model blastocyst development and implantation.Nature. 2022; 601: 600-605https://doi.org/10.1038/s41586-021-04267-8- Keren-Shaul H.
- Kenigsberg E.
- Jaitin D.A.
- David E.
- Paul F.
- Tanay A.
- Amit I.
MARS-seq2.0: an experimental and analytical pipeline for indexed sorting combined with single-cell RNA sequencing.Nat. Protoc. 2019; 14: 1841-1862https://doi.org/10.1038/s41596-019-0164-4- Kunath T.
- Arnaud D.
- Uy G.D.
- Okamoto I.
- Chureau C.
- Yamanaka Y.
- Heard E.
- Gardner R.L.
- Avner P.
- Rossant J.
Imprinted X-inactivation in extra-embryonic endoderm cell lines from mouse blastocysts.Development. 2005; 132: 1649-1661https://doi.org/10.1242/dev.01715- Lancaster M.A.
- Renner M.
- Martin C.-A.
- Wenzel D.
- Bicknell L.S.
- Hurles M.E.
- Homfray T.
- Penninger J.M.
- Jackson A.P.
- Knoblich J.A.
Cerebral organoids model human brain development and microcephaly.Nature. 2013; 501: 373-379https://doi.org/10.1038/nature12517- Lee C.
- Gardner L.
- Turco M.
- Zhao N.
- Murray M.
- Coleman N.
- Rossant J.
- Hemberger M.
- Moffett A.
What is trophoblast? A combination of criteria define human first-trimester trophoblast.Stem Cell Rep. 2016; 6: 257-272https://doi.org/10.1016/j.stemcr.2016.01.006- van Maele-Fabry G.
- Delhaise F.
- Picard J.J.
Evolution of the developmental scores of sixteen morphological features in mouse embryos displaying 0 to 30 somites.Int. J. Dev. Biol. 1992; 36: 161-167- Mandrycky C.J.
- Williams N.P.
- Batalov I.
- El-Nachef D.
- de Bakker B.S.
- Davis J.
- Kim D.H.
- DeForest C.A.
- Zheng Y.
- Stevens K.R.
- Sniadecki N.J.
Engineering Heart Morphogenesis.Trends Biotechnol. 2020; 38: 835-845https://doi.org/10.1016/j.tibtech.2020.01.006- Mittnenzweig M.
- Mayshar Y.
- Cheng S.
- Ben-Yair R.
- Hadas R.
- Rais Y.
- Chomsky E.
- Reines N.
- Uzonyi A.
- Lumerman L.
- et al.
A single embryo, single cell time-resolved model for mouse gastrulation.Cell. 2021; 184: 2825-2842.e22https://doi.org/10.1016/j.cell.2021.04.004- Morgani S.
- Canham M.
- Nichols J.
- Sharov A.
- Migueles R.
- Ko M.
- Brickman J.
Totipotent embryonic stem cells arise in ground-state culture conditions.Cell Rep. 2013; 3: 1945-1957https://doi.org/10.1016/j.celrep.2013.04.034- New D.A.T.
Whole embryo culture and the study of mammalian embryos during organogenesis.Biol. Rev. 1978; 53: 81-122https://doi.org/10.1111/j.1469-185x.1978.tb00993.x- Nichols J.
- Smith A.
Naive and Primed Pluripotent States.Cell Stem Cell. 2009; 4: 487-492https://doi.org/10.1016/j.stem.2009.05.015- Niwa H.
- Toyooka Y.
- Shimosato D.
- Strumpf D.
- Takahashi K.
- Yagi R.
- Rossant J.
Interaction between Oct3/4 and Cdx2 determines trophectoderm differentiation.Cell. 2005; 123: 917-929https://doi.org/10.1016/j.cell.2005.08.040- Parameswaran M.
- Tam P.P.L.
Regionalisation of cell fate and morphogenetic movement of the mesoderm during mouse gastrulation.Dev. Genet. 1995; 17: 16-28https://doi.org/10.1002/dvg.1020170104- Rivron N.C.
- Frias-Aldeguer J.
- Vrij E.J.
- Boisset J.C.
- Korving J.
- Vivié J.
- Truckenmüller R.K.
- van Oudenaarden A.
- van Blitterswijk C.A.
- Geijsen N.
Blastocyst-like structures generated solely from stem cells.Nature. 2018; 557: 106-111https://doi.org/10.1038/s41586-018-0051-0- Seong J.
- Frias-Aldeguer J.
- Holzmann V.
- Kagawa H.
- Sestini G.
- Heidari Khoei H.
- Scholte Op Reimer Y.
- Kip M.
- Pradhan S.J.
- Verwegen L.
- et al.
Epiblast inducers capture mouse trophectoderm stem cells in vitro and pattern blastoids for implantation in utero.Cell Stem Cell. 2022; 29: 1102-1118.e8https://doi.org/10.1016/j.stem.2022.06.002- Shimizu T.
- Ueda J.
- Ho J.C.
- Iwasaki K.
- Poellinger L.
- Harada I.
- Sawada Y.
Dual inhibition of Src and GSK3 maintains mouse embryonic stem cells, whose differentiation is mechanically regulated by Src signaling.Stem Cell. 2012; 30: 1394-1404https://doi.org/10.1002/stem.1119- Sideris I.G.
- Nicolaides K.H.
Amniotic fluid pressure during pregnancy.Fetal Diagn Ther. 1990; 5: 104-108https://doi.org/10.1159/000263555- Sozen B.
- Amadei G.
- Cox A.
- Wang R.
- Na E.
- Czukiewska S.
- Chappell L.
- Voet T.
- Michel G.
- Jing N.
- et al.
Self-assembly of embryonic and two extra-embryonic stem cell types into gastrulating embryo-like structures.Nat. Cell Biol. 2018; 20: 979-989https://doi.org/10.1038/s41556-018-0147-7- Sturm K.
- Tam P.
Isolation and culture of whole postimplantation embryos and germ layer derivatives.Methods Enzymol. 1993; 225: 164-190https://doi.org/10.1016/0076-6879(93)25013-r- Tam P.P.
Postimplantation mouse development: whole embryo culture and micro-manipulation.Int. J. Dev. Biol. 1998; 42: 895-902- Tam P.P.L.
- Snow M.H.L.
The in vitro culture of primitive-streak-stage mouse embryos.J. Embryol. Exp. Morphol. 1980; 59: 131-143https://doi.org/10.1242/dev.59.1.131- Tanaka S.
- Kunath T.
- Hadjantonakis A.K.
- Nagy A.
- Rossant J.
Promotion of trophoblast stem cell proliferation by FGF4.Science. 1998; 282: 2072-2075https://doi.org/10.1126/science.282.5396.2072- Veenvliet J.v.
- Bolondi A.
- Kretzmer H.
- Haut L.
- Scholze-Wittler M.
- Schifferl D.
- Koch F.
- Guignard L.
- Kumar A.S.
- Pustet M.
- et al.
Mouse embryonic stem cells self-organize into trunk-like structures with neural tube and somites.Science. 2020; 370: eaba4937https://doi.org/10.1126/science.aba4937